The CDC Has Just ‘Paused’ The Johnson & Johnson COVID-19 Vaccine Over Blood Clot Concerns
If you were thinking that the 1-dose Johnson & Johnson COVID-19 vaccine sounded promising and planned to get it, everything has changed.
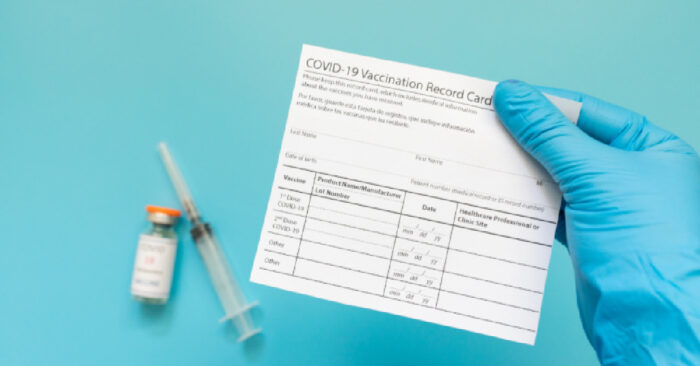
The U.S. FDA and The CDC has pulled the Johnson & Johnson COVID-19 vaccine and placed a pause on it since it has been causing blood clots.
U.S. CDC and FDA are reviewing data involving six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving the J&J vaccine.
The joint statement continued with:
People who have received the J&J vaccine who develop severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider. Health care providers are asked to report adverse events to the Vaccine Adverse Event Reporting System at https://vaers.hhs.gov/reportevent.html.
The U.S. federal distribution channels, including mass vaccination sites, will pause the use of the J&J shot, and states and other providers are expected to follow.
The other two authorized vaccines, from Moderna and Pfizer, make up the vast share of COVID-19 shots administered in the U.S. and are not affected by the pause.
So, there you have it, as soon as we know more, we will update you.