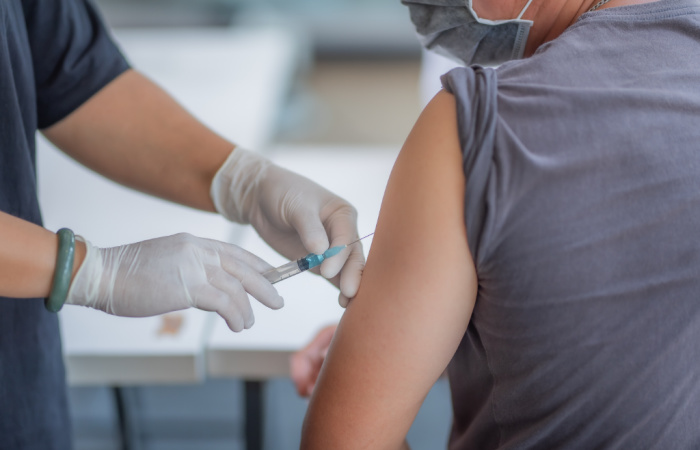
The Johnson & Johnson Single Dose Vaccine Could Be Approved As Soon As This Week
As you might have witnessed yourself, trying to schedule a date and time for the COVID vaccine is like pulling teeth.
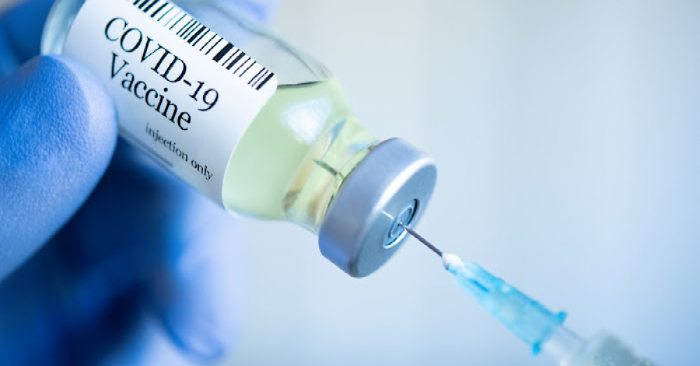
Pfizer-BioNTech and Moderna are the current vaccines being administered throughout the U.S. and now, a third player has been added.
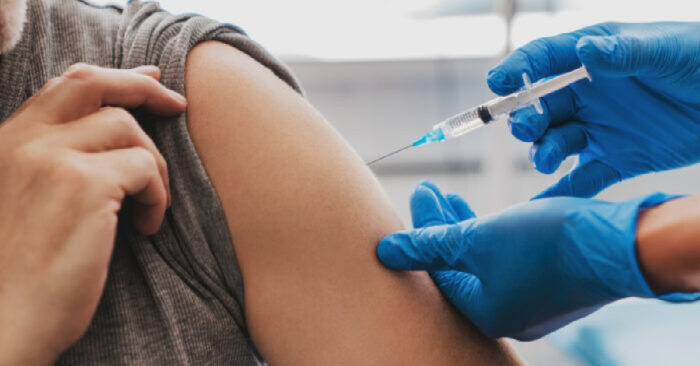
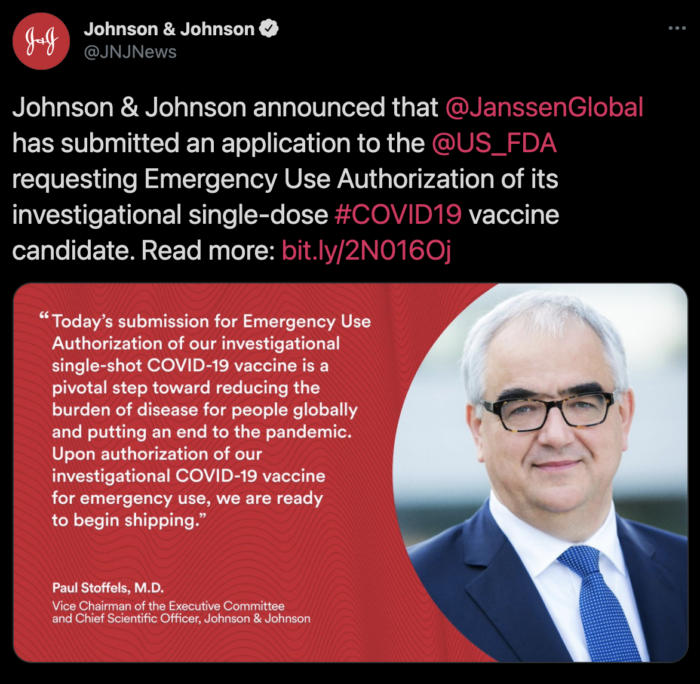
Johnson & Johnson and the FDA recently released a detailed analysis of clinical trials of the vaccine and according to the Food and Drug Administration, Johnson & Johnson’s single dose vaccine meets the requirements for emergency use authorization.
Johnson & Johnson’s vaccine unlike Pfizer-BioNTech and Moderna requires only one shot to be injected which means only one trip to the doctor folks!
The good news is that this vaccine was found to be 66% effective against all symptomatic COVID cases and 85% effective at preventing severe disease; the great news is that this vaccine was also found to be effective against all known variants of the virus.

Preliminary data also shows that the vaccine may help prevent asymptomatic infections which could be a major helping hand to slow the spread of the virus.
The next question is when and if the vaccine will be approved and that depends on the public hearing scheduled this Friday.
If the public hearing is in support of the authorization, the FDA could make their decision as early as this Friday evening.
Johnson & Johnson says it could produce 20 million doses over the next few weeks and deliver 100 million by the end of June, which means more people can get vaccinated.
Johnson & Johnson has also submitted their vaccine to the World Health Organization and other public health organizations for approval in hopes to distribute the vaccine globally which would be incredibly helpful.