There’s A New COVID Vaccine Clinical Trial For Pregnant Women
So many people are getting the COVID-19 vaccine, and that is hopefully going to be SUCH a good thing.

There is one group of the population, however, that the World Health Organization (The WHO) and the Center For Disease Control And Prevention (The CDC) haven’t been able to come to a conclusive decision on whether they should get the vaccine or not.

That group is Pregnant women.
Pregnant women are at higher risk of developing severe COVID-19, and many public health officials have recommended some women in high-risk professions take coronavirus vaccines even without proof they are safe for them.
Reuters

But, everyone wants to know, is the vaccine safe for the unborn? Is it safe for the mommies?

Obviously, the vaccine is so new, nobody has been able to answer any of these questions.
Pfizer and BioNTech are taking care of that, and launching a vaccine clinical trial on pregnant women.
I know. It makes me nervous, too.
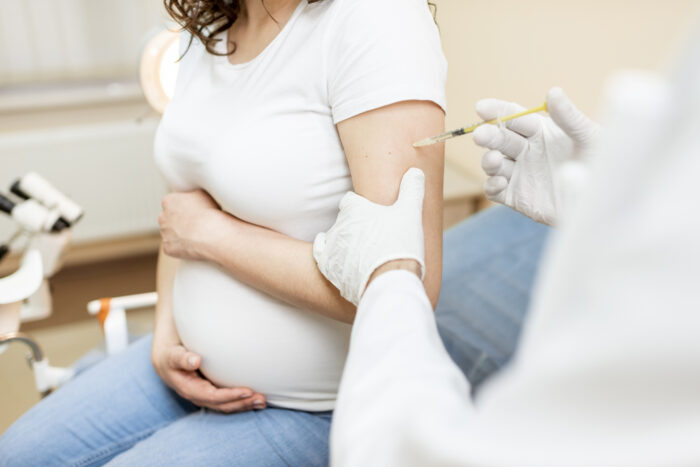
This marks the first trial of its kind in the U.S.
Pfizer-BioNTech are going to be giving 4,000 pregnant woman the vaccine, and they are going to assess things like the safety of the vaccine, tolerability of the vaccine, and how well the vaccine produces immunity in these women.

The women in this study are all going to be between 24 and 34 weeks gestation, and all the participants are over the age of 18.
They will be given a series of 2 shots — 21 days apart — just like the vaccine in the general population.

The new study will test pregnant women aged 18 and older in the United States, Canada, Argentina, Brazil, Chile, Mozambique, South Africa, the UK and Spain.
Reuters
There WILL be a placebo group, as they do in trials, and that group will have the option to get the ACTUAL vaccine after they give birth.

Good luck, and happy immunity to all the pregnant women in the trial.
We will let you know if and when they update us on the vaccine trial.








